-
Electrochemistry and the Neuron – an Analytical Review
Abstract
Electrochemistry is the process of oxidation-reduction reactions that produce electrochemical reactions and is the intersection between chemistry and electricity. In electrochemistry, electrons are transferred as a result of oxidation-reduction reactions, meaning that a chemical reaction results in electrical movement. Electrochemistry is an applicable concept in a multitude of fields, one being neural activity. Oxidation-reduction reactions mediate neural communication, playing a crucial role in the facilitation of overall neural processes. An equation to quantify and define the electrochemical equilibrium between the concentration gradient and the electrical potential for a certain ion is the Nernst equation. The regulation of the concentration gradient and electrical potential is done through reversal potential3, and can be calculated through given concentrations of the particular ion.
- Introduction of the Nernst Equation and Action Potential
The Nernst equation is utilized as a quantitative demonstration of concentrations of an ion that exists on either side of the membrane7. The potential difference in concentration of these ions is classified as the “voltage”, and can be measured when the membrane is in equilibrium. With this said, an action potential is a rapid change in the voltage, or concentration of a particular ion, across a neuron membrane. The function of the action potential is to transmit information across the brain by utilizing the axons located within the white matter of the brain. This process of action potential can be determined by the relative ratio of ions existing on either side of the membrane, which is where the Nernst Equation can be applied. Referring to the images below, the first equation is the general Nernst equation, while the equation on the right is applied in action potential within neurons. Action potentials occur within 1 millisecond, making it a very fast reaction2. In addition to this, action potentials are considered as an all-or-nothing action, so the voltage of the neurons have to meet a certain threshold in order for the information to be transmitted across neurons. A figure depicting this concept is found in Section III.
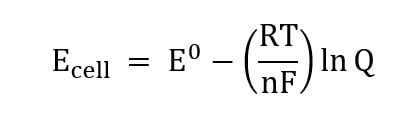

Figures 1 and 2 – the general Nernst Equation and the Nernst Equation in Action Potential, Respectively.
- Analysis of the Nernst Equation
As can be seen in the first equation on the left, the voltage of the cell (Ecell) is equal to the standard voltage potential (E0) subtracted by the entirety of the gas constant (8.314 J/mol-1k-1) times temperature (Kelvin) divided by the number of electrons transferred multiplied by Faraday’s constant (96485 C/mol) and finally multiplied by the natural log of the Q, the concentration of products divided by the concentration of reactants. The standard voltage is obtained by determining the standard reduction potential at the anode and subtracting this value by the standard reduction potential of reduction at the cathode. Often, the value of RT in the original Nernst equation is simplified to 0.0591/n since R and F are constants, while the temperature (T) is typically 298 K, which is 25 degrees Celsius. The derivation of the Nernst equation comes from Gibbs Free Energy (ΔG), which is the change in the enthalpy – the change in the amount of heat in a process. Simply put, this Gibbs Free Energy equation contains the state functions of thermodynamics values. The original equation is written as ΔG = ΔGo + RTlnQ, and this equation can be rewritten by substituting known values that are equal to one another, which are ΔG = -nFEcell and ΔGo = -nFEocell. The resulting equation is -nFEcell = -nFEocell + RTlnQ. Upon dividing both sides by -nF, the Nernst Equation is created.
- The Integration of the Nernst Equation in Neuron Physiology A) Introduction to the Neural Membrane
The concentrations that mediate the voltage in a neuron are sodium (Na+), potassium (K+), and chloride (Cl–)1. The extracellular region has a high concentration of Na+ and Cl–, while there is a low concentration of K+. Intracellularly, there is only a high concentration of K+ and a low concentration of Na+ and Cl–. These concentrations are mediated through an active ion pump, with the most common one being the Na+/K+ pump. This pump requires energy, and Na+ tends to depolarize the cell (makes the intracellular area more positive), K+ hyperpolarizes the cell (makes the intracellular area more negative), and Cl– hyperpolarizes the cell. These differences in concentrations across a semipermeable membrane creates an electrical potential, which can be portrayed through the equation mentioned previously1:

B) Determining Voltage Potential and Exploring Driving Force
The Nernst equation focuses on individual ion concentrations, so the Nernst equation is typically applied towards Na+, K+, and Cl–. The concentrations exist as milliMolar and the concentration of the selected ion outside of the membrane is divided by the concentration of the ion inside the membrane. As with the typical application of Nernst equation, the greater amount of the ion inside the cell signifies a lower voltage. The reversal potential of Na+ – the value where there is no net flux – has a reversal potential of +59mV, -65mV for Cl–, and -80mV for K+3. This means that these are the values that these ions try to reach in order to reach equilibrium potential. When there is depolarization across the neuron and there is a signal sent for the voltage-gated channels to open4, the difference between the membrane potential and the reversal potential of a certain ion will determine the electrochemical driving force (Vdf)6. This driving force is what causes the ions to move down its own electrochemical gradient. This magnitude of the electrochemical driving force determines how far an ion is from reaching an equilibrium.
Vdf = Vm – Veq
C) Occurrence of Depolarization – Signal Transmission
Image taken from https://jackwestin.com/resources/mcat-content/nerve-cell/action-potential
As can be seen in the figure above, the typical voltage of the resting potential is -0.70 mV, meaning that there are more sodium ions inside the cell than there are potassium ions within the cell. Moreover, this means that an external force is required to create the potential and send it through the neuron. With this positive value of the voltage, then the change in Gibbs Free Energy (ΔG) is positive, which means that the reaction of a neuron is not spontaneous. When there is a depolarization inside the cell, voltage-gated Na+ channels open, allowing Na+ into the cell (depolarizing the cell) and therefore continuing the propagation of the signal. This creates the voltage potential that can then be quantified through the Nernst equation. At the maximum voltage (peak action potential), the voltage is typically measured to be +30mV. When the neuron goes through the process of repolarization, the intracellular area is becoming more negative, meaning that ions such as the Na+ is exiting and making their way to the extracellular space. This flux of ions is not precise, however, so the membrane typically overshoots and reaches a “hyperpolarized” state, meaning that there are too many polarizing ions intracellularly and has reached a voltage below its typical equilibrium resting state.
- How Action Potential is Measured in a Neuron
In order to determine the voltage of the neuron, two microelectrodes are typically inserted into the neuron, with one connected to a stimulator while the other one is connected to a voltmeter to measure the voltage2. The electrode stimulator is often connected to a battery, which corroborates the idea that an external energetic force needs to be applied to the battery in order for an action potential to occur. This electrode stimulator can either add a positive voltage or negative voltage to the neuron, showing the depolarizations and polarizations of the neuron.
- Applying the Nernst Equation in Action Potential A) Benefits of its Applications
The Nernst equation, as has been covered, is able to be used to measure the exact value of the change of the equilibrium potential. As a result, a person is able to determine the cell potential even under non-standard conditions. At a broader application, the complexities of the brain are able to start being understood using this Nernst Equation. Decades of research have been in work to determine which areas of the brain mediate certain actions. For instance, motor control can be linked to the motor cortex within the brain, but to potentially figure out lesser known connections that dictate a certain behavior/action, calculating the polarization – or lack thereof – of a neural membrane in a certain area of the brain can be used as a way to corroborate that a certain area is responsible for a given action/behavior. Because of this, the Nernst Equation can be used in research and discovering neuroanatomical regions responsible for mediating certain behaviors.
B) Shortcomings of its Applications
The downfall of the Nernst Equation is that it only shows the concentrations of one ion that is altering its concentration across the membrane, but multiple ions play a role in membrane potential5. This means that, although the equation can be applied effectively for finding the equilibrium, the equation is not as reliable when there are multiple voltage-gated channels that are responsible for mediating the polarization – and therefore the transfer of a message – of a neuron5. Because scientists are aware of the limitations of the Nernst Equation, an equation was created for when a membrane is permeable to more than one ion. As previously mentioned, K+ and Na+ play crucial roles in the polarization and depolarization, respectively. This equation is known as the Goldman-Hodgkin and Katz (GHK) equation2.
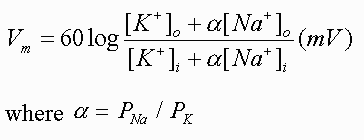
The GHK Equation, with alpha signifying the ratio of Na+ permeability to K+ permeability.
This equation transforms into the Nernst Equilibrium potential if there is a lack of presence of either ion within this equation. The permeability portion (α) is important in determining voltage since a membrane can be one-hundred times more permeable to K+ than Na+ or vice versa, meaning that much more of that given ion would travel across the membrane, making it an uneven diffusion of the ions even if the concentrations of these ions are the same2.
- Concluding Remarks
As has been explained throughout the paper, the Nernst Equation is an effective way to measure the voltage of the neurons during an action potential. The action potential is the process by which neurons transmit information, which is an integration of both chemical and electrical signaling. The action potentials are able to be compared to an electrolytic cell, because it requires an external input of energy into the neuron in order for an action potential to occur. Since specific ions are able to either polarize or depolarize the cell – which dictates the action potential – the Nernst Equation measures the concentrations of an ion being diffused against a selectively permeable membrane and the initial voltage of the cell (which typically hovers around -70mV) to calculate the voltage of the membrane. The voltage of the cell can be calculated whether in equilibrium or not in equilibrium. Moreover, the driving force of a particular ion can also be considered to determine which space – whether extracellular or intracellular – the ion will try to go to in order to maintain equilibrium. However, the Nernst Equation is not as applicable when a membrane is selectively permeable to more than one membrane, so the GHK Equation is applied instead in this circumstance. It is important to know the voltage of certain neurons in order to determine which neuroanatomical regions mediate certain behaviors/actions. To conclude, the Nernst Equation is crucial to determine the voltage of a membrane when this membrane is only selectively permeable for one ion.
- References
- Braun, J. (n.d.). Theoretical neuroscience I lecture 6: Synaptic conductances. Theoretical Neuroscience Part I. https://bernstein-network.de/wp-content/uploads/2021/02/01_Lecture-01-Nernst-equation.pdf
- Byrne, J. H. (2021). Resting potentials and action potentials (Section 1, Chapter 1) Neuroscience Online: An electronic textbook for the Neurosciences: Department of Neurobiology and Anatomy – the University of Texas Medical School at Houston. Resting Potentials and Action Potentials (Section 1, Chapter 1) Neuroscience Online: An Electronic Textbook for the Neurosciences | Department of Neurobiology and Anatomy – The University of Texas Medical School at Houston. https://nba.uth.tmc.edu/neuroscience/m/s1/chapter01.html#:~:text=One%20can%20use%20the%20Nernst,changes%20by%20about%2015%20mV
- Fitzakerley, J. (2014, September 20). 2014 Ion Channel Physiology. Equilibrium potentials. https://www.d.umn.edu/~jfitzake/Lectures/DMED/IonChannelPhysiology/MembranePotentials/EquilibriumPotentials.html#:~:text=Equilibrium%20(or%20reversal)%20potentials&text=Erev%20can%20be%20calculated,K%2B%20is%20~%2D88%20mV.&text=for%20a%20given%20ion%2C%20the,R%20%3D%20gas%20constant
- Grider, M. H., Jessu, R., & Kabir, R. (2022, May 15). Physiology, action potential – statpearls – NCBI bookshelf. Physiology, Action Potential. https://www.ncbi.nlm.nih.gov/books/NBK538143/
- National Library of Medicine. (2001). Molecular biology of the cell – NCBI bookshelf. Voltage-Gated Ion Channels. https://www.ncbi.nlm.nih.gov/books/NBK21054/
- PhysiologyWeb. (2015, February 15). Electrochemical driving force acting on ions – resting membrane potential. Resting Membrane Potential. https://www.physiologyweb.com/lecture_notes/resting_membrane_potential/resting_membrane_potential_electrochemical_driving_force_acting_on_ions.html#:~:text=The%20driving%20force%20is%20the,ion%20is%20from%20its%20equilibrium
- TeachMePhysiology. (2021, August 23). Resting membrane potential – nernst – generation. Resting Membrane Potential. https://teachmephysiology.com/nervous-system/synapses/resting-membrane-potential/#:~:text=Nernst%20Equation,-The%20Nernst%20equation&text=where%20Vm%20%3D%20equilibrium%20potential,inside%20the%20cell%20%5Bmol%5D
-
Exploring Neuromodulation

Brain Mapping with Electrodes Introduction
Closed-Loop Neuromodulation, also known as an intelligent neuromodulation, is a flexible neural device that sends a signal in response to a biomarker. This focused delivery of a stimulus to neural tissue whose intent is to affect neural or physiological processes helps researchers understand more about human physiology1. A certain biomarker threshold has to be met in order for the closed-loop neuromodulation to be activated, meaning that this device acts as a Responsive Neurostimulation (RNS)2. RNS differs from Deep Brain Stimulation (DBS) since DBS is created to reduce the frequency of brain malfunction while RNS targets specific malfunctions at the time of their occurrence. If you want to learn more about DBS, take a look at my other blog, Deep Brain Stimulation – A Neurorehabilitation Method for Parkinson’s Patients. Closed-loop neuromodulation encapsulates RNS, DBS, and Vagus Nerve Stimulation (VNS). An early example of closed-loop neuromodulation is its application in refractory epilepsy. Refractory epilepsy is when the seizures are unable to be controlled by medication or when the condition is too severe, warranting the therapeutic use of closed-loop neuromodulation. Once implanted, when a seizure begins in the patient, the electrodes stimulate certain areas of the brain to stop the episode3. In the beginning, an open-loop neuromodulation was used, a system whose response is dependent on a timed algorithm rather than in response to a neural stimulus. These systems are set by a neurologist and require multiple appointments. As can be presumed, the closed-loop neuromodulation is much more effective in its response since it is able to immediately respond to the biomarker stimulus instead of only being activated at scheduled times2.
RNS and VNS
Responsive Neurostimulation (RNS) is a method that monitors electrical irregularities in the brain. It is an invasive method where a neurostimulator is implanted into the brain and is connected to wires that extend into areas of the brain where seizures occur. When triggered, it delivers a brief pulse of electrical stimulation through the leads. RNS is a great rehabilitative method, because it can be used to supplement epilepsy treatment and can be removed at any point5. How this device works as a treatment for epilepsy is that it disrupts the “electrical storm” that occurs in the brain when a seizure occurs. This intervention alleviates the amount of electrical stimulation and may even circumvent a seizure altogether6. Vagus Nerve Stimulation (VNS) intends to alter the activity of nerves by having an implanted device that sends mild pulses of electrical energy to the brainstem through the vagus nerve. Once it reaches the brainstem, the electrical charge disseminates across the brain and changes how brain cells work. VNS is believed to improve blood flow in critical areas of the brain, alter the electrical stimulations that occur when a person is having a seizure, and increase the level of neurotransmitters that control seizure development7.
Types of Neuromodulation
The algorithm within a closed-loop neuromodulation focuses on when a stimulation should be activated and how strong this stimulus should be. In current research, this device could be responsive to changes in neurochemical composition, such as potassium, sodium, calcium, or other neurotransmitters. As this can be adjusted, this system can be personalized for each individual. Neuromodulation can be separated into five different types of systems: scheduled intermittent, continuous, responsive, adaptive, and complete closed-loop. Scheduled intermittent is a form of an open-loop system, where the stimulation is sent at scheduled times as opposed to being sent in response to a biomarker4. A continuous neuromodulation is another open-loop system where the stimulus is continuously sent despite the physiological factors. A responsive neuromodulation is a partial closed-loop system where the stimulation is initiated when a certain physiological biomarker threshold is met. However, the device is still pre-set. An adaptive neuromodulation monitors only one biomarker and is a closed-loop system. This system is able to adjust its levels of stimulus according to the biomarker reaction. Lastly, a complete closed-loop neuromodulation is a system that monitors multiple biomarkers while also adapting to the biomarker reaction. In order for the adaptive and complete closed-loop neuromodulation to be able to operate autonomously, they must undergo training. This training can focus on biomarkers such as neurotransmitters and neurochemicals, which will then be analyzed by neuromodulation systems in order to create an appropriate reaction4.
Potential of Neuromodulation
In recent years, the development of the closed-loop neuromodulation system has taken precedence over the open-loop systems due to developments in response to physiological biomarkers and its ability to be personalized. However, open-looped systems continue to demonstrate its efficacy as a rehabilitative device. A research paper published in 2016 provided data showing that, when an intermittent open-looped neuromodulation device was implanted in the thalamus of patients, it reduces tics in patients with Tourette Syndrome8. Another benefit of neuromodulation is that they act as an alternative treatment to epilepsy when patients are drug-resistant. Because neuromodulation is able to either increase or decrease nerve activity, the device regulates neural activity. With this being said, it can alleviate persistent pain, spasticity, movement disorders, bowel/bladder dysfunction, spinal injury, ischaemia, and other impairments. Neuromodulation has the potential to regulate many ailments pertaining to the nervous system, making it one of the more effective treatments10. I believe that as studies progress, it will be a definitive intervention for epilepsy, movement disorders such as Parkinson’s Disease, and may even have the ability to counter the prominent symptoms of dementia-related illnesses like short-term memory loss and brain atrophy. Although the illnesses that neuromodulation could resolve are vast, there are still both ethical and clinical issues that still need to be addressed.
Issues of Applying Closed-Loop Neuromodulation in the Clinical Setting
As closed-loop neuromodulation is a recently-developed neurorehabilitative measure, there are still many factors to be studied when applying this device in the clinical setting. Namely, knowing where to place the electrodes, the effects of adjusting the detector and stimulation parameters, and the overall lack of knowledge of the long-term effects of using this advice. Additionally, electroclinical correlation and dissociation, patient concerns about device capabilities, clinician opportunities and burdens, and data ownership and access are other notable issues that need to be addressed. Electroclinical correlation and dissociation refers to the device’s limited spatial sampling and limited storage capacity. These limitations have resulted in recordings of epileptic episodes being deleted or the device is unable to detect the origination of a seizure. This leads to a conflict as to how much freedom an epileptic patient may have, as they may be restricted from driving from faulty recordings of seizures. Patients also are not trustworthy of the data it records due to these malfunctions. To add on, with open-loop systems, specialists are required to modify the device weekly, placing a strain on their time. Lastly, the data recorded can be accessed by other institutions, which could be perceived as a breach in privacy9.
Cited Sources
1. Stavros, Z. (2019, November 1). Closed-loop neuromodulation in physiological and Translational Research. Closed-Loop Neuromodulation in Physiological and Translational Research. Retrieved May 10, 2022, from https://pubmed.ncbi.nlm.nih.gov/30559253/
2. Parastarfeizabadi, M., & Kouzani, A. (2017, August). Overview of open-loop DBS. Advances in Closed-Loop Deep Brain Stimulation Devices. Retrieved May 11, 2022, from https://www.researchgate.net/figure/Overview-of-open-loop-DBS-a-versus-closed-loop-DBS-b-In-open-loop-DBS-a-neurologist_fig3_319068029
3. Kam, K. (2021, April 28). Help When Epilepsy Treatment Doesn’t Work. Refractory epilepsy: Causes, symptoms, treatment, and more. Retrieved May 11, 2022, from https://www.webmd.com/epilepsy/refractory-epilepsy
4. Mirza, K. B., Golden, C. T., Nikolic, K., & Toumazou, C. (2019, August 20). Closed-loop implantable therapeutic neuromodulation systems based on neurochemical monitoring. Frontiersin. Retrieved May 10, 2022, from https://www.frontiersin.org/articles/10.3389/fnins.2019.00808/
5. Unknown. (2020, October 7). Responsive neurostimulation. ucsfhealth.org. Retrieved May 10, 2022, from https://www.ucsfhealth.org/treatments/responsive-neurostimulation
6. Unknown. (n.d.). Responsive neurostimulation. Responsive Neurostimulation | Neurological Surgery | University of Pittsburgh. Retrieved May 14, 2022, from https://www.neurosurgery.pitt.edu/centers/epilepsy/responsive-neurostimulation
7. Unknown. (n.d.). Vagus nerve stimulation (VNS): What it is, uses & side effects. ClevelandClinic.org. Retrieved May 14, 2022, from https://my.clevelandclinic.org/health/treatments/17598-vagus-nerve-stimulation-vns
8. Rossi, J. P., Opri, E., Shute, J. B., Molina, R., Bowers, D., Ward, H., Foote, K. D., Gunduz, A., & Okun, M. S. (2016, August). Scheduled, intermittent stimulation of the thalamus reduces tics in tourette syndrome. Parkinsonism & related disorders. Retrieved May 12, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4969169/
9. Hegde, M., Chiong, W., & Rao, V. R. (2021, April 27). New ethical and clinical challenges in “closed-loop” neuromodulation. Neurology. Retrieved May 12, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8166428/
10. Neuromodulation frequently asked questions – faqs. International Neuromodulation Society. (2013, July). Retrieved May 12, 2022, from https://www.neuromodulation.com/neuromodulation-faqs# -
Deep Brain Stimulation – A Neurorehabilitation Method for Parkinson’s Patients
Parkinson’s Background and Prevalence
Parkinson’s is a progressive neurodegenerative disorder that affects movement. As a result, those with Parkinson’s tend to have debilitating symptoms such as tremors, slowed movement, rigid muscles, and writing changes1. When looking at the population as a whole, over ten million people are living with Parkinson’s Disease, and these numbers are rising as a result of aging populations, increasing longevity, declining smoke rates, and a by-product of industrialization2. In a study, it was noted that there is a great correlation between age increase and an increase in motor rate progression, decreased levodopa responsiveness, postural impairment, worsened gait, and increase in cognitive impairment as compared to younger patients. Therefore, the general human population increasing their life longevity leads to a greater chance of developing Parkinson’s Disease and the disease becoming more prominent. With these increased cases, it has been taken upon by scientists to research an approach to alleviate the symptoms of Parkinson’s3. Current interventions to alleviate the symptoms of Alzheimer’s include Carbidopa-levodopa, dopamine agonists, and MAO B inhibitors1.
Parkinson’s at the Molecular Level
The essential cause of Parkinson’s Disease is the death of dopaminergic neurons – neurons responsible for synthesizing the dopamine neurotransmitter. Both enzymatic and non-enzymatic oxidation of dopamine generates oxygen species that are reactive, resulting in apoptotic cell death in dopamine neurons. Neurotoxins such as MPTP, TCE, and PERC also contribute to cell death5.. This cell death occurs particularly in the substantia nigra, a location in the brain that is responsible for the production of dopamine. Because this dopaminergic neuron loss is what results in Parkinson’s, administration of the aforementioned medications such a Carbidopa-levodopa is an effective way to counter Parkinson’s symptoms since Carbidopa is a decarboxylase inhibitor that is able to prolong the life of levodopa – which converts to dopamine4. All prescribed medications are efforts to create more dopamine for the brain, but Deep Brain Stimulation seeks to circumvent the abnormal functions of the brain of a Parkinson’s patient by delivering electrical impulses inside the brain.
Deep Brain Stimulation
Deep Brain Stimulation, originating in the late 1980’s, is a revolutionary discovery that currently works as a treatment for movement disorders. Deep Brain Stimulation is an invasive procedure in which electrodes are implanted into certain areas of the brain, allowing for an electrical current to stimulate the brain. The intention of Deep Brain Stimulation is to interrupt the abnormal signaling that occurs in Parkinson’s patients, such as tremors, by sending an electrical impulse. The electrodes are specifically implanted in target areas of the basal ganglia – a subcortical nuclei primarily responsible for motor control – such as the subthalamic nucleus or globus pallidus internus. The subthalamic nucleus is in charge of motor regulation, while the globus pallidus internus is responsible for inhibiting the ventral lateral nucleus and ventral lateral nucleus of the thalamus. To elaborate, the function of this portion of the brain is regulation of motor movement, since the areas it inhibits are centers for motor control7. This is done to change the activity between the direct and indirect basal ganglia pathways to promote motor movement without the dopamine6.
Deep Brain Stimulation and the Direct Basal Ganglia Pathway
In the direct basal ganglia pathway, the glutamate neurons project from the thalamus to motor regions in the cerebral cortex. These excitatory projections stimulate movement and are regulated by the globus pallidus internus and substantia nigra pars reticulata. These regulations are known as inhibitory projections, which inhibit thalamic neurons and suppress movement. From the cerebral cortex, the stimulation continues to the striatum through the corticostriatal pathway, where neurons in the striatum are activated and release GABA to the globus pallidus internus and substantia nigra pars reticulata. This inhibits the inhibitory projections, allowing for thalamus signals to flow freely to the cerebral cortex. The substantia nigra pars compacta is believed to control the striatum by releasing dopamine, creating further movement8. Thus, with the electrical stimulation of the subthalamic nucleus or globus pallidus internus, motor function can be regulated since the electrodes will control the inhibitory projections and the excitatory projections within the direct basal ganglia pathway. As a result, the most prominent symptoms of Parkinson’s – tremors, rigid muscles, and others – are regulated through these electrode stimulations.
Here is a Youtube video that explains this concept more simply and provides diagrams.
2-Minute Neuroscience: Direct Pathway of the Basal Ganglia
Deep Brain Stimulation Compared to other Treatments
Deep Brain Stimulation only resolves motor issues within Parkinson’s patients and is not a cure to Parkinson’s. However, there are no treatments that are able to cure Parkinson’s, they only alleviate the symptoms that interfere with daily life. As mentioned before, the combined drug Carbidopa-levodopa is an effective treatment that works to prevent nausea. Other than this symptom relief, though, this medication is not enough to relieve the more pressing symptoms of Parkinson’s. There is a potential treatment that has been going through clinical trials, though. This method is a non-invasive ultrasound treatment that utilizes ultrasonic energy and is aimed at the globus pallidus. Rather than having to be put under anesthesia to have electrodes implanted in the brain, the patient is able to stay awake and alert throughout the treatment process. The patient is able to talk to the doctors and as a result, immediate adjustments could be made. After only a short time on this treatment, patients report their symptoms being relieved. Tremors, rigidity, and dyskinesia are all said to be greatly relieved from this novel treatment9.
Final Thoughts
Deep Brain Stimulation has been an effective treatment throughout the decades, but the advent of more non-invasive methods, such as the ultrasound stimulation treatment, will be favored as more research is conducted and will likely take over Deep Brain Stimulation as a common treatment for Parkinson’s since the chances of complications occurring is much lower. In my own perspective, I believe that ultrasound stimulation is becoming an effective therapeutic treatment for a multitude of health issues. Having interned at a laboratory that explored sonogenetics – the non-invasive manipulation of neurons and other cells expressing exogenous protein channels – I had the opportunity to observe the process of stimulating cells with ultrasound and analyzing its effects firsthand. Utilizing ultrasound creates the potential to regulate systems that have become faulty or abnormal, such as the case with the direct basal ganglia pathway in Parkinson’s patients and the glucose regulation pathway in diabetic patients.
Organizations and More
If you are interested in donating to organizations that provide relief to Parkinson’s patients, feel free to check out the links below.
If you want to navigate through other charities yourself and find the most reliable, Charity Navigator provides plentiful data for thousands of organizations.
Charity Navigator – Assessment of Charities
Parkinson’s Foundation:
Parkinson’s Foundation – General Gift
This Foundation has been rated as one of the most reliable charities. As seen on the website, your money contribution will be used to provide free educational materials, send out care kits, fund exercise classes, fund research, and more.
Parkinson’s Association:
Their mission is to “optimize quality of life for people affected by Parkinson’s disease through programs and services that enhance MIND, MOVEMENT, and MORALE.” This Association provides a variety of activities, programs, and opportunities for the community to help those with Parkinson’s.
Parkinson’s Association – General Page
Parkinson’s Association – General Information Brochure
References
1. Mayo Foundation for Medical Education and Research. (2022, March 24). Parkinson’s Disease. Mayo Clinic. Retrieved April 20, 2022, from https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/symptoms-causes/syc-20376055
2. Dorsey, E. R., Sherer, T., Okun, M. S., & Bloem, B. R. (2018). The emerging evidence of the parkinson pandemic. Journal of Parkinson’s disease. Retrieved April 20, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6311367
3. Gilberto, L. (2007, September). The Relationship of Parkinson Disease with Aging. Jama Neurology. Retrieved April 20, 2022, from https://jamanetwork.com/journals/jamaneurology/fullarticle/794410#
4. U.S. National Library of Medicine. (2022). Levodopa and carbidopa: Medlineplus Drug Information. MedlinePlus. Retrieved April 20, 2022, from https://medlineplus.gov/druginfo/meds/a601068.html
5. Naoi, M., & Maruyama, W. (n.d.). Cell death of dopamine neurons in aging and Parkinson’s disease. Mechanisms of aging and development. Retrieved April 20, 2022, from https://pubmed.ncbi.nlm.nih.gov/10656535/
6. Sonne, J., Reddy, V., & Beato, M. R. (n.d.). Www.ncbi.nlm.nih.gov. Neuroanatomy, Substantia Nigra. Retrieved April 20, 2022, from https://www.ncbi.nlm.nih.gov/books/NBK536995/
7. Lanciego, J. L., Luquin, N., & Obeso, J. A. (2012, December 1). Functional neuroanatomy of the basal ganglia. Functional Neuroanatomy of the Basal Ganglia. Retrieved April 20, 2022, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3543080/
8. Andrușca, Alexandru MD, P. D. (2022, March 13). Basal ganglia pathways. Kenhub. Retrieved April 21, 2022, from https://www.kenhub.com/en/library/anatomy/direct-and-indirect-pathways-of-the-basal-ganglia
9. Kotz, D. (2022, March 2). A new era for parkinson’s disease treatment. A New Era for Parkinson’s Disease Treatment. Retrieved April 21, 2022, from https://www.umaryland.edu/news/archived-news/march-2022/a-new-era-for-parkinsons-disease-treatment.php
TheGreyAnalysis
A blog centered on current neurtechnological innovations and self-improvement.
This blog explores current neurotechnological innovations and dives into self-improvement methods supported by science.
Check out some of the most recent blogs below.
To learn more about the structure of this blog, check out my introduction page!
Deep Brain Stimulation (DBS) – A Neurorehabilitation Method for Parkinson’s Patients.
Exploring Neuromodulation.
Scroll Down Further to See my Posts!